CLP
Classification, Labelling and Packaging of substances and mixtures (CLP)
PAA and CLP
The CLP Regulation (EC) No 1272/2008 for substances and mixtures harmonises the hazard classification and labelling of chemical substances and mixtures in the European Union and the European Economic Area.
CLP aligns the European Union with the Globally Harmonised System (GHS) for the classification and labelling of substances. The purpose of CLP is to ensure a high level of protection of human health and the environment by (i) harmonizing the criteria for classification of substances and mixtures, (ii) harmonizing the rules for labelling and packaging of hazardous substances, (iii) obliging manufacturers, importers and downstream users to classify and label & package accordingly their substances and mixtures placed on the market, (iv) providing an obligation to notify ECHA thereof and (v) establish a list of substances with harmonised classification.
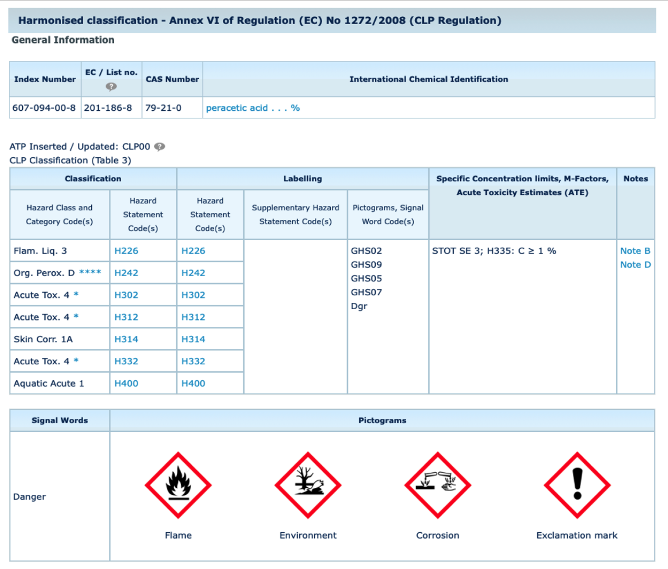
PAA and CLP
PAA is harmonized classified according to Annex VI of the CLP Regulation. The role of PAA Europe is, for example, to safeguard that any proposal for harmonized classification is made on the basis of sound scientific knowledge and in accordance with the available scientific data.
https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/24363
LATEST NEWS – Finland has made its intentions known to submit a proposal for adjustment of the current CLH proposal. PAA Europe will be actively following and involved during this procedure, this to evaluate the impact on the PAA market and to ensure all available and necessary information reaches the relevant parties and stakeholders.
